Analysis of Recalled Products Regulated by the FDA was performed by pintegriti.
- Source: FDA website “Recalls, Market Withdrawals, & Safety Alerts”
- Data: 1127 entries from Sep 08, 2017 to Dec 28, 2023
- Data analysis and visualization tool: Python in Excel, Pandas, Matplotlib libraries
The FDA data table originally categorizes products into 115 distinct types, which have been reorganized into 9 consolidated product categories for streamlined analysis.
- Food: food, beverages, ice cream, fruit, frozen dairy, seafood, snack, infant formula, bakery, bottled water
- Drugs: prescription drugs, OTC drugs
- Drugs_sanitizer: hand sanitizer
- Devices: medical devices, lab tests, dental
- Animal: animal food and beverage
- Animal_drug
- Tobacco
- Dietary: dietary supplements, nutritional supplements, vitamins
- Cosmetics: skin care, hair, deodorant
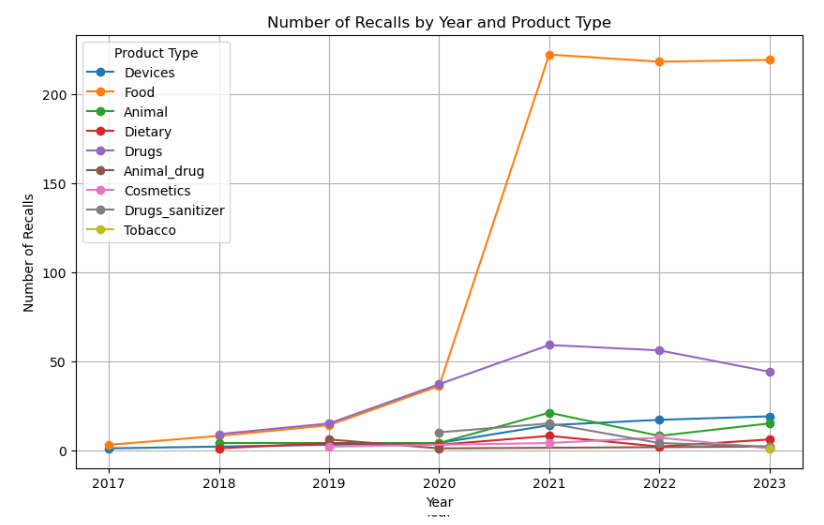
Figure 1 displays the annual trend of recalls, categorized by product type, offering a chronological overview of recall patterns over the years.
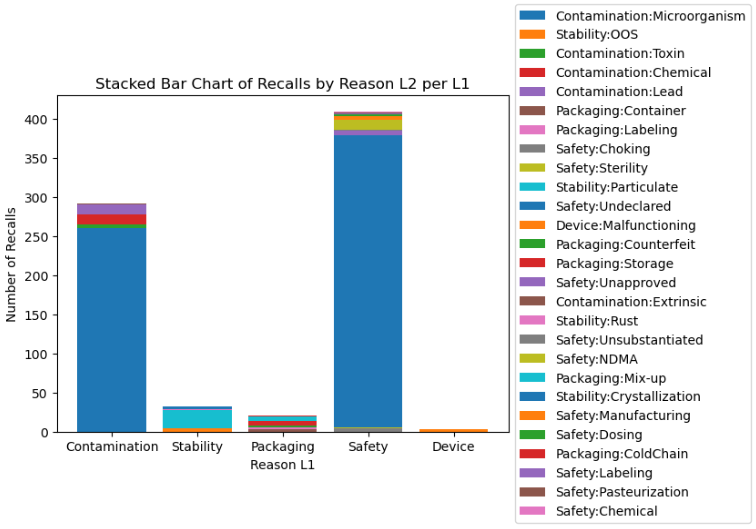
Recall reasons are categorized into two distinct levels. Level 1 recall reasons encompass several classifications, including Safety, Contamination, Stability, Packaging, and Device. Subsequently, under each Level 1 category, Level 2 recall reasons are further classified as follows:
- Safety: Undeclared, Sterility, Pasteurization, Manufacturing, Choking, Unsubstantiated, Unapproved, NDMA, Dosing
- Contamination: Microorganism, Chemical, Extrinsic, Lead, Toxin
- Stability: Particulate, Crystallization, OOS (out of specification), Rust
- Packaging: Container, Counterfeit, Storage, Mix-up, Labeling, Cold Chain
- Device: Malfunctioning
Figure 2 presents an annual analysis of recalls, categorized by Level 1 reasons. It reveals that the predominant factors leading to the recall of Drugs and Foods are predominantly related to Safety and Contamination concerns.
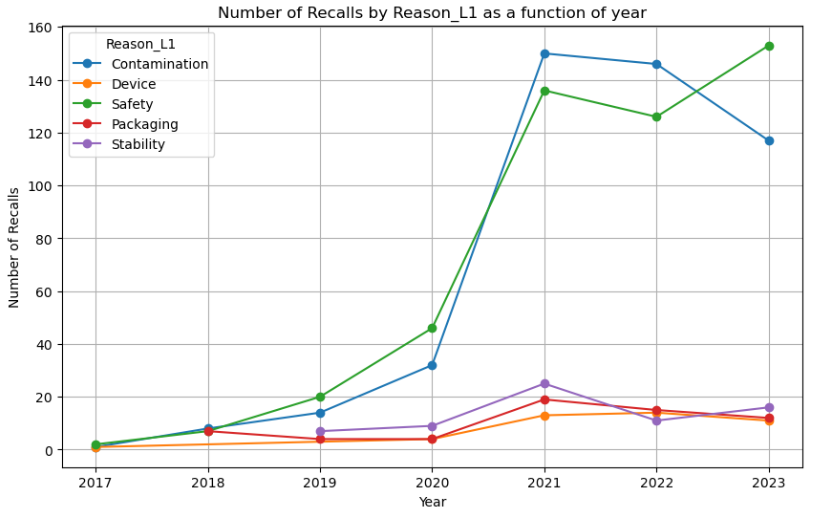
Figure 3 shows the bar chart Level 1 reasons of recalls per product type.
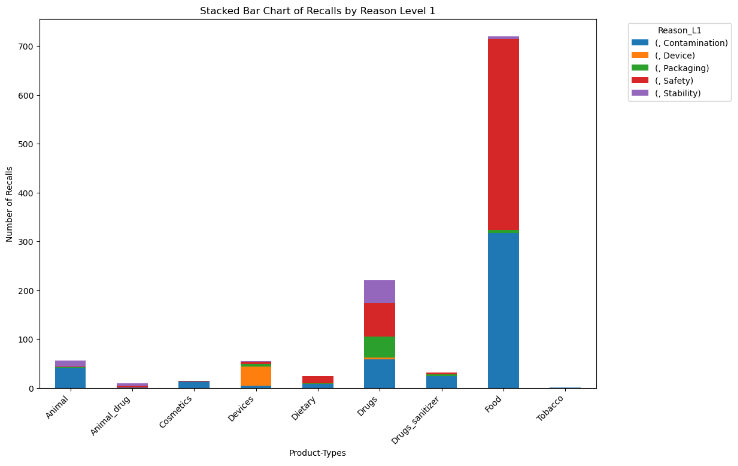
Figure 4 illustrates the distribution of Level 2 recall reasons within each Level 1 category. Notably, under the ‘Contamination’ category, microorganisms emerge as the primary contaminants in products, while the use of undeclared ingredients stands out as a leading cause for product recalls.
Notes.
- OOS: out of specification of impurity, potency, color, etc.
- Chemical: benzene, methanol, other API
- Container: breakage, compromised seal, sanitizer container resembles drink containers
- Labeling: incorrect or missing information
- Undeclared: undeclared ingredients
- Storage: stored outside of labeled temperatures
- Unapproved: unapproved drugs, ingredients, and devices
- Extrinsic: glass, metal or other foreign materials
- Unsubstantiated: unsubstantiated information
- NDMA: N-Nitrosodimethylamine Impurity
- Mix-up: product mix-up
- Manufacturing: insanitary manufacturing conditions, improper process
- Dosing: incorrect dosing cups or graduation markings
- Cold Chain: distributed at freezing temperature