Figure 1 shows the overall drug discovery and development process.
The comprehensive review encompasses a meticulous examination of the entire drug development process, with a particular emphasis on the critical stages extending from First Human Dose (FHD) to the product’s market launch. This in-depth analysis scrutinizes each phase for efficiency, regulatory compliance, and alignment with best practices.
During the review, the transition from the FHD through to the successive phases—Phase I (safety and dosage), Phase II (efficacy and side effects), Phase III (confirmation of effectiveness, monitoring of adverse reactions from a larger patient group), and ultimately to the submission for regulatory approval—is meticulously assessed. Each step is evaluated to ensure that it meets the rigorous safety and efficacy standards set by health authorities.
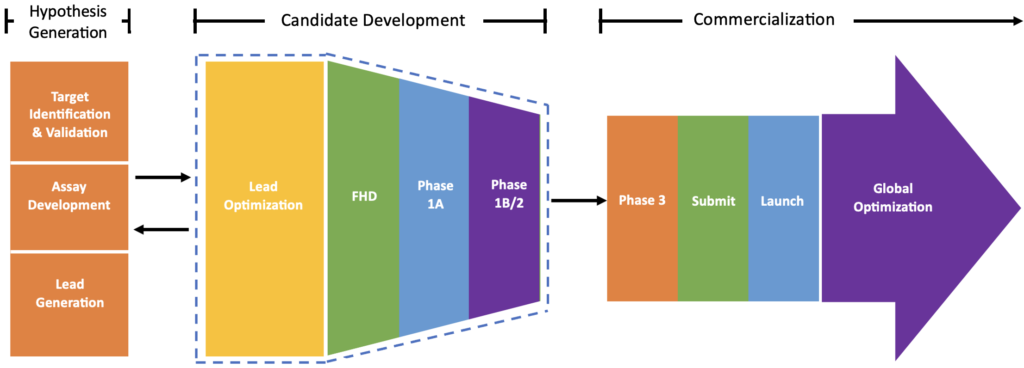
Figure 1. Overall drug discovery and development process (note: the schematic has been recreated by Pintegriti. source: https://www.pghr.org/post/what-makes-drug-discovery-and-development-so-difficult)
- Average cost to develop one drug: ~1.3 billion (source: Wikipedia)
- Average time to develop one drug: 10 – 15 years (source: PhRMA). ~8 years from FHD to Launch
- Number of compounds to develop one drug: 5,000 to 10,000 compounds
- Hypothesis Generation
- Lead Optimization
- FHD Preparation
- Phase 1A: one in ten candidates reaching the market
- Phase 1B/2
- Phase 3/Submit/Launch
- Global Optimization
